Clinical Performance
The performance of the CoviDx™ SARS-CoV-2 Rapid Antigen Test was determined by assessing nasal and nasopharyngeal swabs from symptomatic individuals within 5 days of symptom onset who were suspected of COVID-19.CoviDx Results vs. RT-PCR1
CoviDx SARS-CoV-2 Rapid Antigen Test was compared to an Emergency Use Authorized molecular (RT-PCR) test for SARS-CoV-2. The samples were collected during high and low prevalence states and are inclusive of newly
emerging variant strains including the UK (B.1.1.7.), South African and Denmark variants.
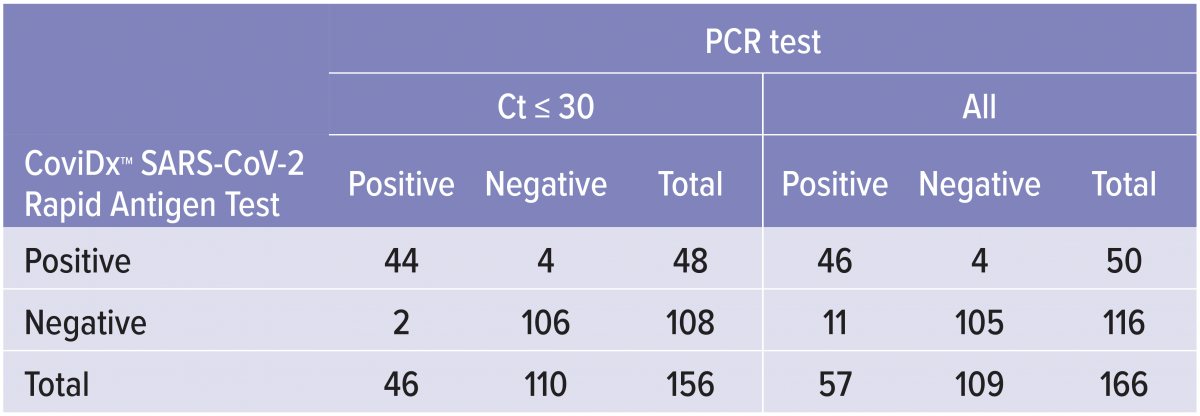
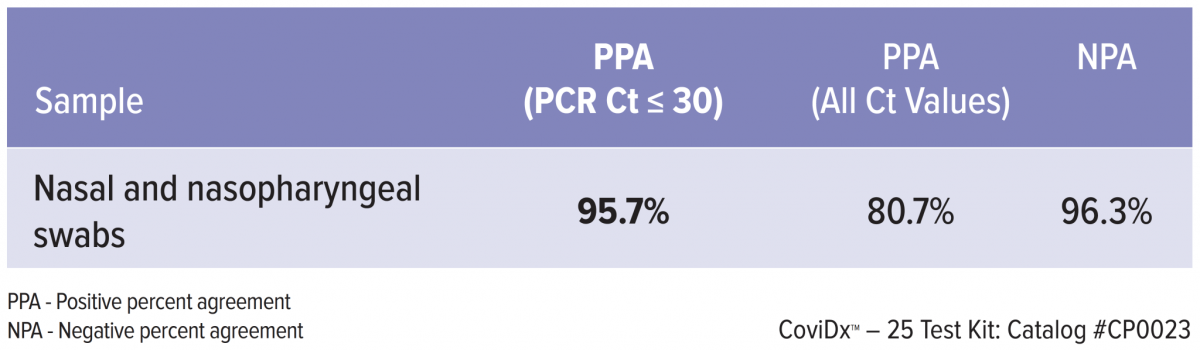
CoviDx SARS-CoV-2 Rapid Antigen Test results were stratified by RT-PCR cycle threshold (Ct) to show assay performance in the context of the viral load present in the clinical sample.
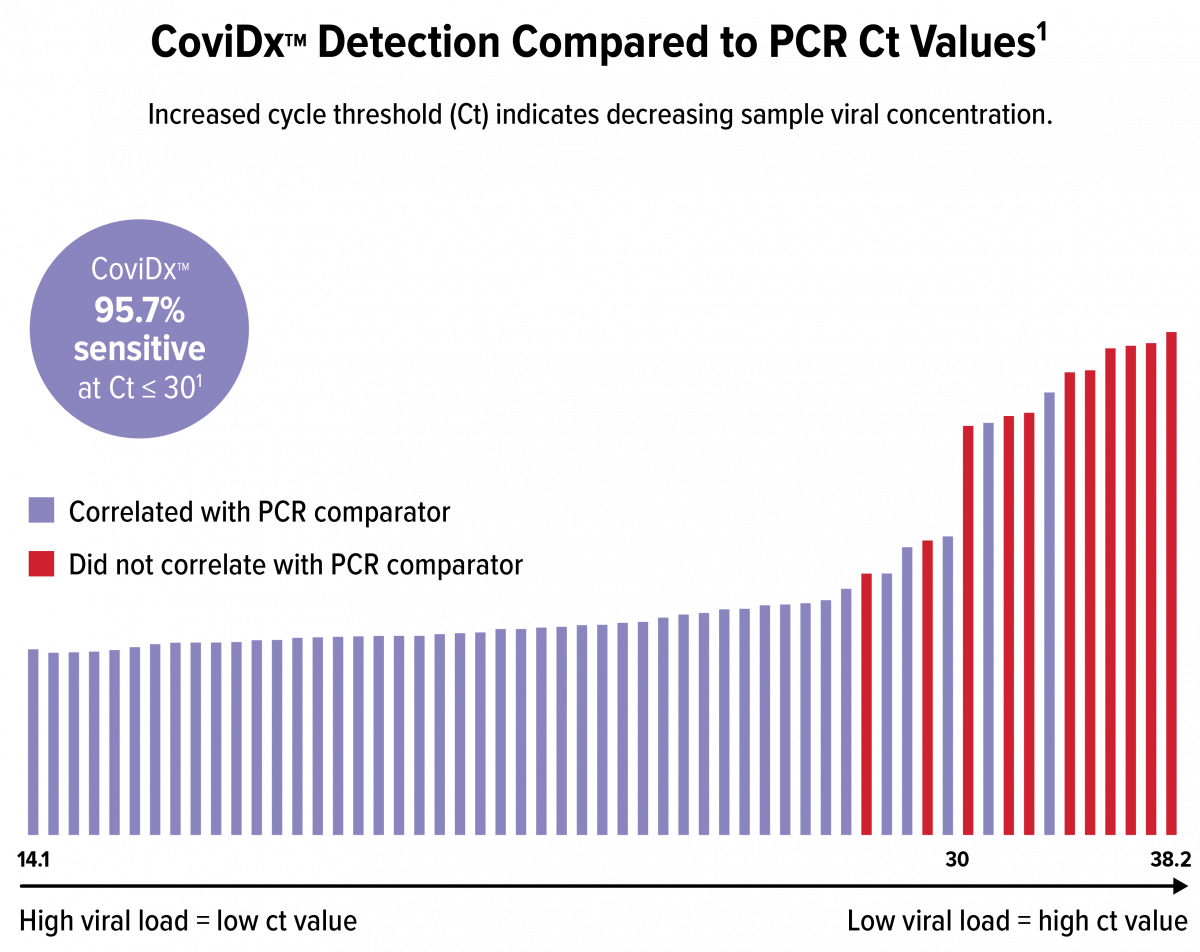

CoviDx PPA vs. RT-PCR1

CoviDx SARS-CoV-2 Rapid Antigen Test results were stratified by RT-PCR cycle threshold (Ct) to show assay performance in the context of the viral load present in the clinical sample.

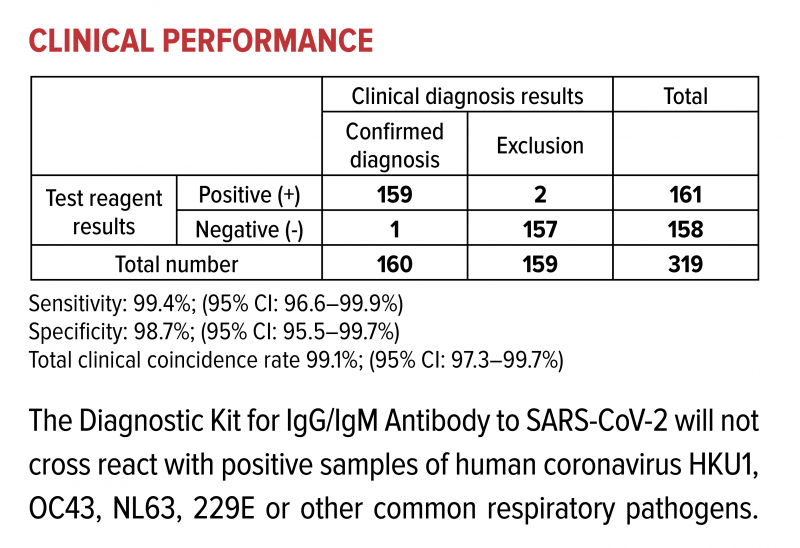


Contact us to order or learn more about the CoviDx SARS-CoV-2 Rapid Antigen Test.


