Simple Test Procedure
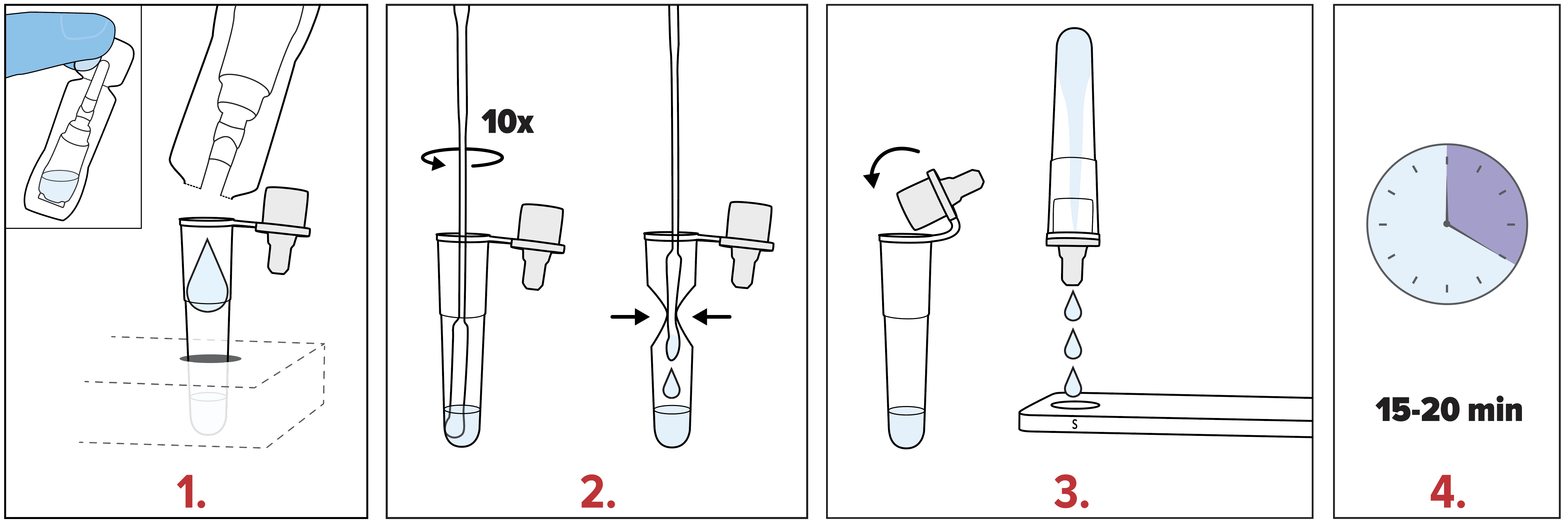

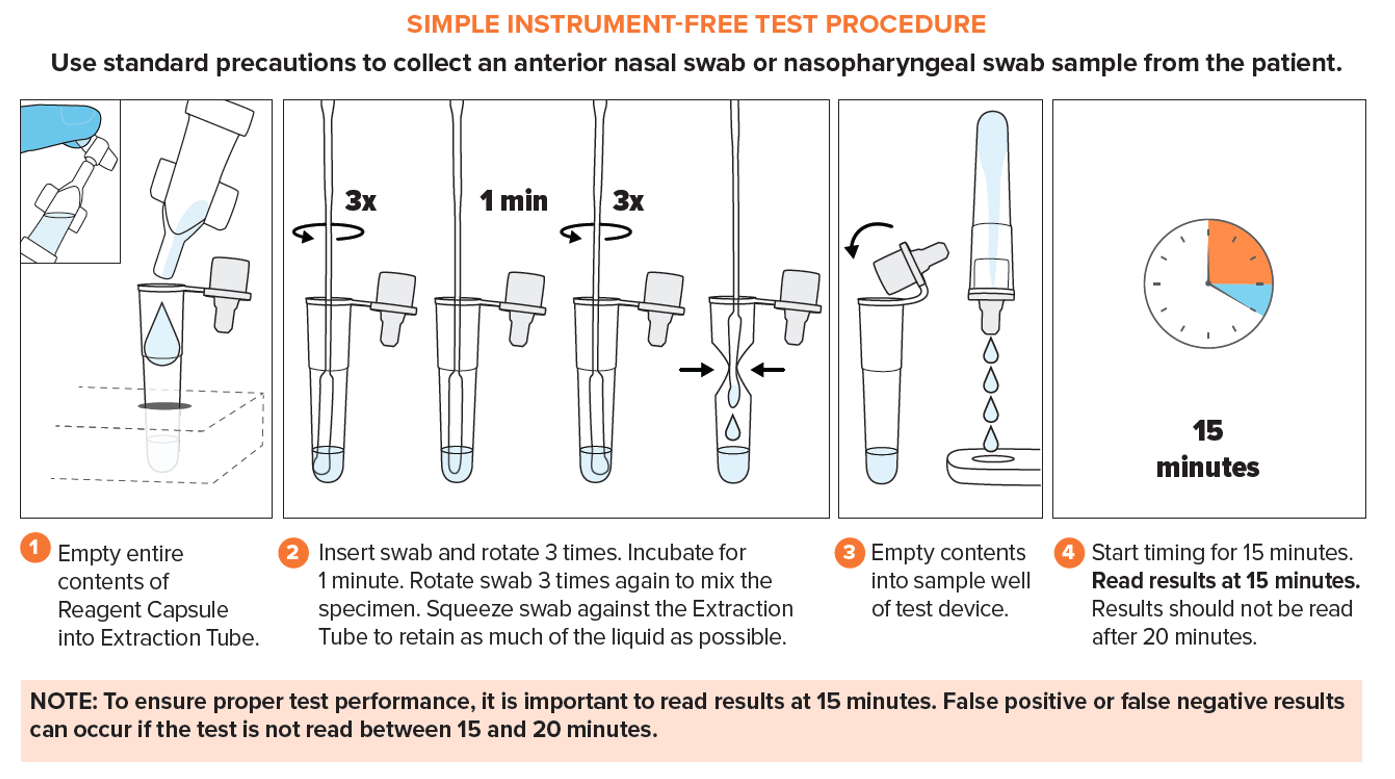
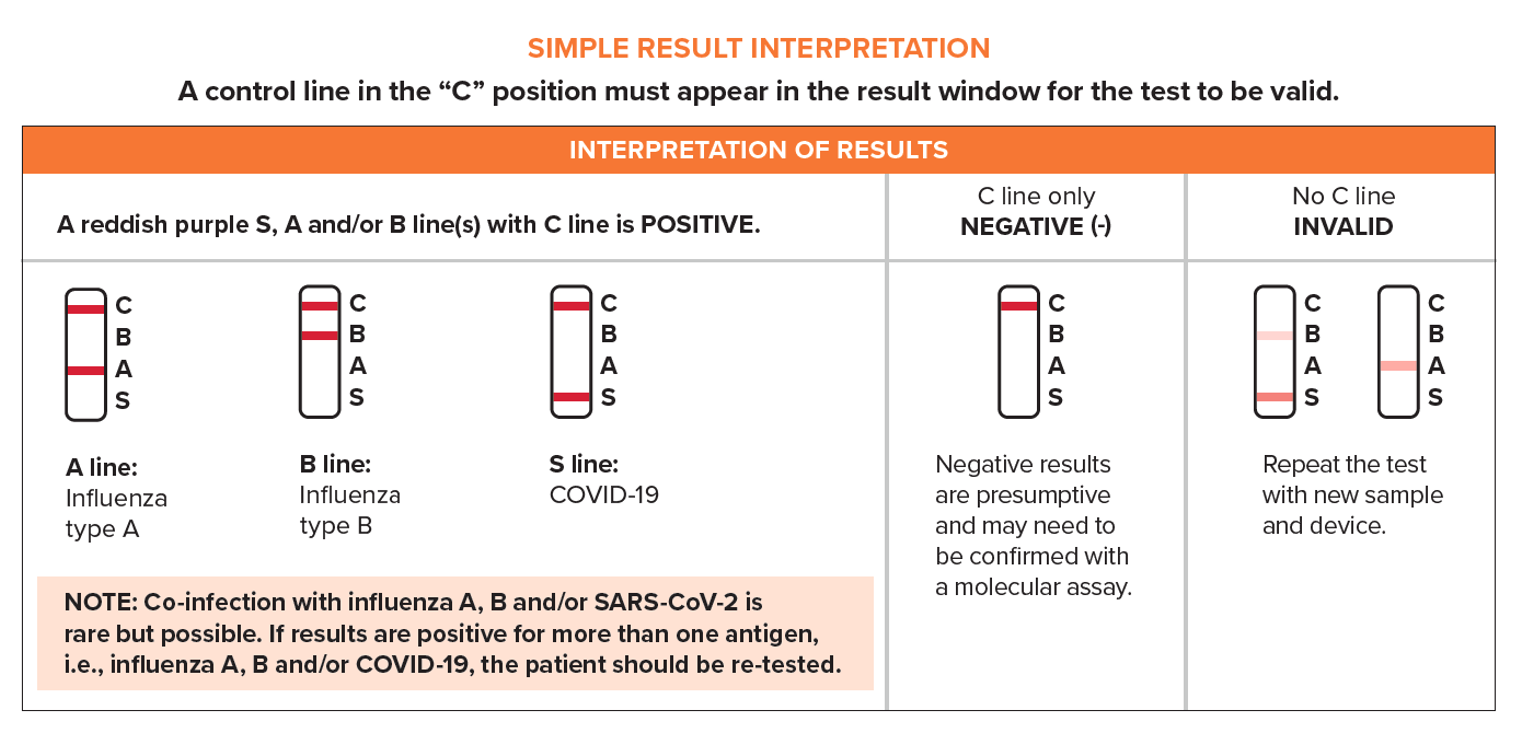
- ViraDx SARS-Cov-2/Flu A+B Rapid Antigen Test is authorized for use in Canada under Health Canada Interim Order (IO).
- For in vitro diagnostic use.
- Rx only.
- Refer to the Package Insert for complete instructions. Read the complete test procedure, including recommended Quality Control procedures, before performing the test.
- All clinical specimens must be at room temperature before beginning the assay.
- Expiration date: Check expiration on each individual test package or outer box before using. Do not use any test past the expiration date on the label.
- ViraDx SARS-CoV-2/Flu A+B Rapid Antigen Test is not available in the United States.


Contact us to learn more about the ViraDx 3-in-1 rapid antigen test.

