Benefits of using ViraDx
- Efficient: 1 sample – 3 results to aid in a differential diagnosis at the point of care
- Rapid: actionable results in 15 minutes to help improve patient management decisions during the patient visit
- Instrument-free: user-friendly test procedure for non-lab settings
- Accurate:1
- COVID-19: Sensitivity 93.4%; Specificity 100%
- Flu A: Sensitivity 91.4%; Specificity 95.7%
- Flu B: Sensitivity 87.6%; Specificity 95.9%

| Time to results | 15 minutes |
| Sample type | Anterior nasal or nasopharyngeal swab specimen |
| Interpretation | Visually read (no instrument required) |
| Method | Lateral flow |
| Storage conditions | 2 to 30°C (35 to 86°) |
Influenza (flu) is a highly contagious acute viral infection of the respiratory tract. It is a communicable disease easily transmitted from person to person through aerosol droplets excreted when sneezing and coughing. There are two main types of influenza – type A and type B.2
Early differential diagnosis of influenza type A or type B can allow for proper treatment. Early diagnosis and treatment are of particular value in a clinical setting where an accurate diagnosis can assist the healthcare professional with the management of influenza patients who are at risk for complications.3
COVID-19 is an infectious disease caused by the SARS-CoV-2 virus. Similar to influenza, the virus can spread from an infected person’s mouth or nose ranging from larger respiratory droplets to smaller aerosols.4 COVID-19 symptoms can range from mild to severe.
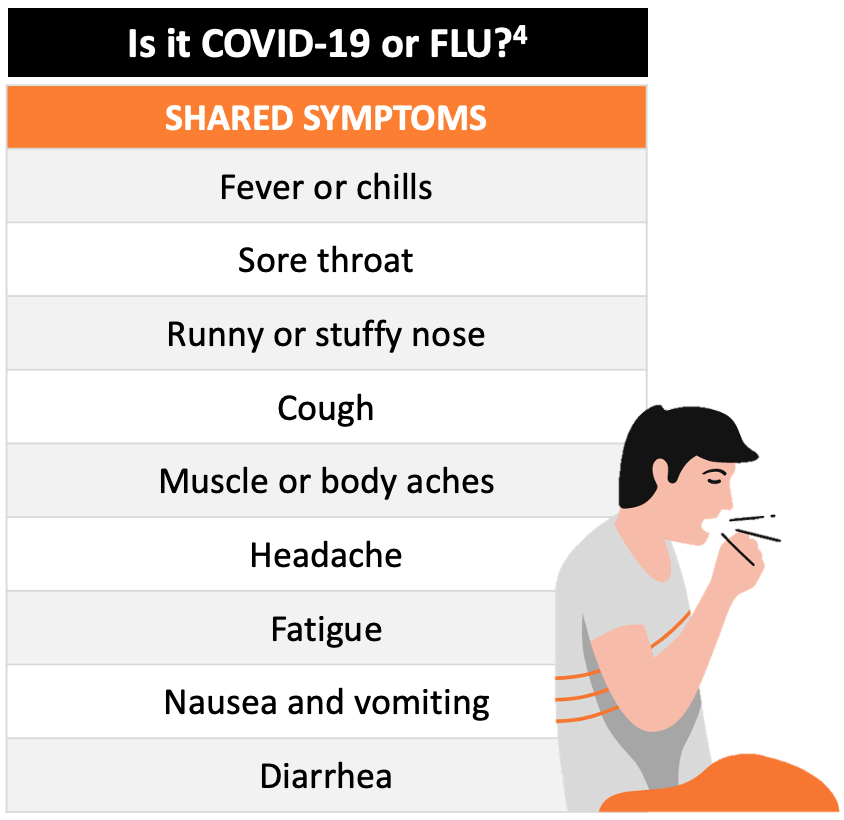
As the early symptoms of COVID-19 are like those of seasonal influenza type A or B, a rapid, point-of-care test that can help support the differential diagnosis of COVID-19, influenza A and B is urgently needed.
- ViraDx SARS-Cov-2/Flu A+B Rapid Antigen Test is authorized for use in Canada under Health Canada Interim Order (IO).
- For in vitro diagnostic use.
- Rx only.
- ViraDx SARS-CoV-2/Flu A+B Rapid Antigen Test is not available in the United States.


Contact us to learn more about the ViraDx 3-in-1 rapid antigen test.

